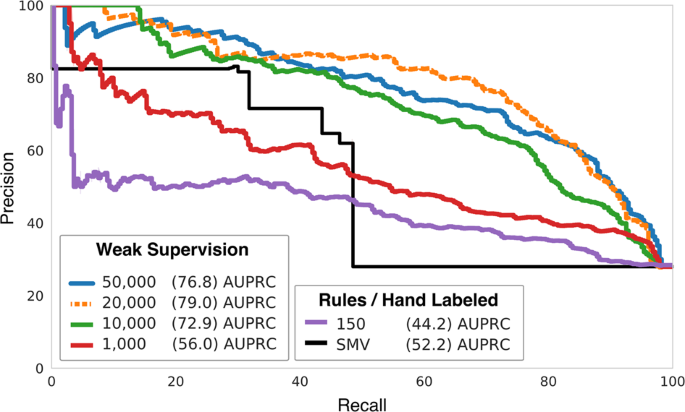
PLOS Neglected Tropical Diseases: The Art of Writing and Implementing Standard Operating Procedures (SOPs) for Laboratories in Low-Resource Settings: Review of Guidelines and Best Practices

Fillable Online erasmus ankara edu 1 COOPERATIONPROJECT INFORMATION Discipline Academic field (ISCED 2013) 0421 Law 0488 Business, administration and law, interdisciplinary programmes - erasmus ankara edu Fax Email Print - pdfFiller
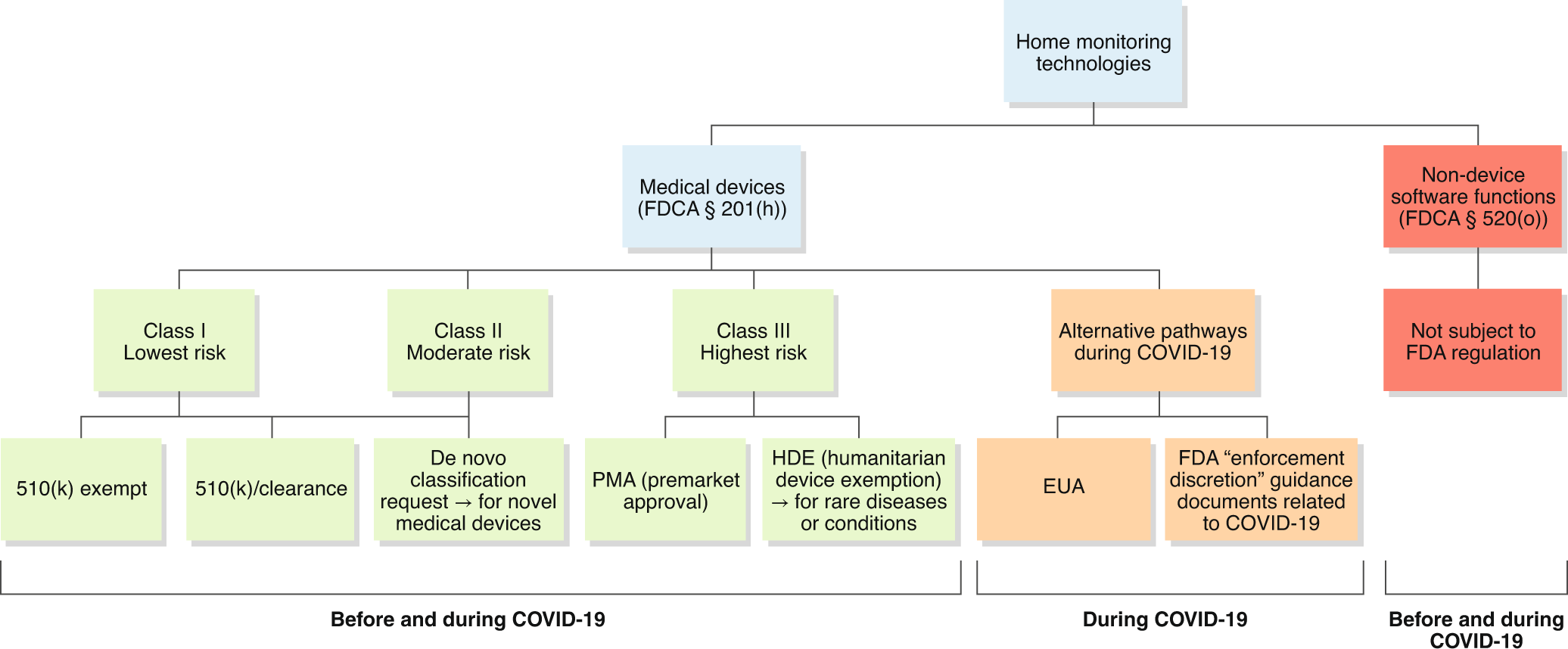
Regulatory, safety, and privacy concerns of home monitoring technologies during COVID-19 | Nature Medicine

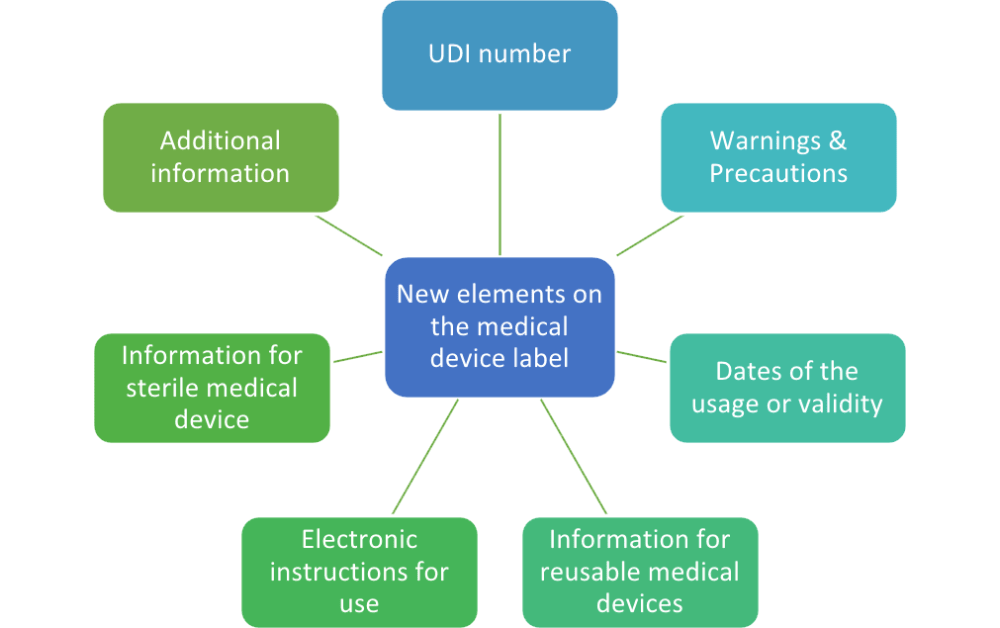
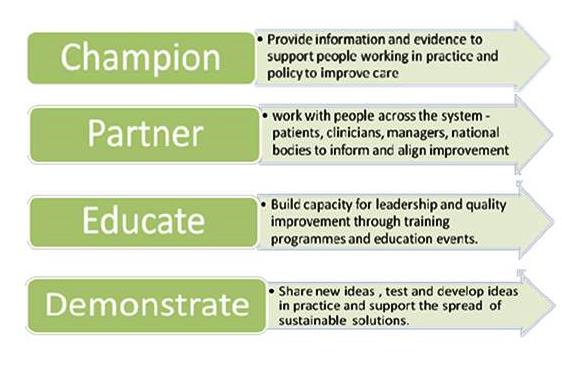
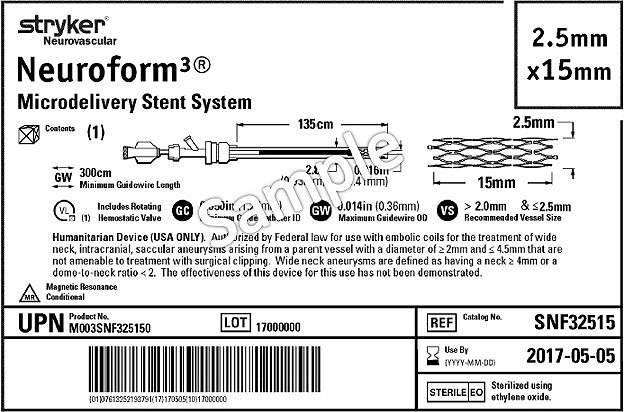
FDA Guidance on Reprocessing Medical Devices: Criteria 1-3 for Reprocessing Instructions | RegDesk